Search
Granulopoiesis

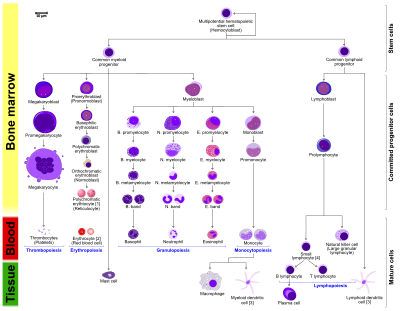
Granulopoiesis (or granulocytopoiesis) is a part of haematopoiesis, that leads to the production of granulocytes. A granulocyte, also referred to as a polymorphonuclear leukocyte (PMN), is a type of white blood cell that has multi lobed nuclei, usually containing three lobes, and has a significant amount of cytoplasmic granules within the cell. Granulopoiesis takes place in the bone marrow. It leads to the production of three types of mature granulocytes: neutrophils (most abundant, making up to 60% of all white blood cells), eosinophils (up to 4%) and basophils (up to 1%).
Stages of granulocyte development
Granulopoiesis is often divided into two parts;
1) Granulocyte lineage determination and
2) Committed granulopoiesis.
Granulocyte lineage determination
Granulopoiesis, as well as the rest of haematopoiesis, begins from a haematopoietic stem cells. These are multipotent cells that reside in the bone marrow niche and have the ability to give rise to all haematopoietic cells, as well as the ability of self renewal. They give rise to either a common lymphoid progenitor (CLP, a progenitor for all lymphoid cells) or a common myeloid progenitor, CMP, an oligopotent progenitor cell, that gives rise to the myeloid part of the haematopoietic tree. The first stage of the myeloid lineage is a granulocyte - monocyte progenitor (GMP), still an oligopotent progenitor, which then develops into unipotent cells that will later on form a population of granulocytes, as well as a population of monocytes. The first unipotent cell in granulopoiesis is a myeloblast.
Committed granulopoiesis
Committed granulopoiesis consists of maturation stages of unipotent cells. The first cell that starts to resemble a granulocyte is a myeloblast. It is characterized by large oval nucleus that takes up most of the space in the cell and very little cytoplasm. The next developmental stage, a promyelocyte, still has a large oval nucleus, but there is more cytoplasm in the cell at this point, also cytoplasmic granules are beginning to form. The development of granules continues with the next stage, a myelocyte. At this point, the nucleus is starting to shrink. At the stage of a metamyelocyte the cell nucleus is becoming kidney-shaped and it becomes even more bent in the stage of a band cell. The maturation is finished with the emergence of a segmented nucleus that is specific for a mature granulocyte.
Regulation of granulopoiesis
Transcriptional regulation
The maturation of granulocytic precursors is tightly regulated at transcriptional level. Granulocyte lineage determination is regulated by expression of C/EBPα, which is necessary for the transition from CMPs to GMPs and levels of PU.1, that drive the differentation from GMPs to monocytes (high PU.1 levels) or to granulocytes (low PU.1 levels). Committed granulopoiesis is regulated by C/EBPε and GFI-1, these two transcriptional factors are important for terminal granulocyte differentiation. Other transcriptional factors that regulate granulopoiesis are: CBF, MYB, SMAD4 and HOX genes.
Regulation by cytokines
Granulopoiesis is also regulated by cytokines to a certain extent. The main cytokines driving granulopoiesis are: GM-CSF (formation of GMPs from CMPs), G-CSF (commitment to the granulocyte lineage, formation of myeloblasts from GMPs), IL-3 (enhances the production of GM-CSF and G-CSF) and SCF. These are secreted by other haematopoietic cells in the bone marrow or at the site of inflammation as well as epithelial and endothelial cells.
Types of granulopoiesis
Steady state granulopoiesis
Steady state granulopoiesis is a term used to describe the normal daily production of granulocytes. Granulocytes are short lived cells (their lifespan is between 6 and 8 hours) with a high cell turnover. The number of granulocytes produced every day is between 5 and 10 x 1010. The master regulator of steady state granulopoiesis is C/EBPα. It restricts the cell cycle of immature cells by inhibition of CDK2 and CDK4 and promotes granulocytic differentiation. Steady state production of granulocytes is activated after the engulfment of apoptotic granulocytes by tissue macrophages.
Emergency granulopoiesis
Emergency granulopoiesis is a fundamental hematopoietic mechanism activated during acute infections or inflammatory conditions, leading to a swift increase in granulocyte production, especially neutrophils, in the bone marrow. This process is essential for enhancing the innate immune system's capability to confront pathogen invasions effectively. Hematopoietic stem cells (HSCs) undergo significant transcriptional reprogramming in response to emergency conditions, transitioning from a lymphoid-biased state towards a myeloid-biased identity, thereby aligning the hematopoietic system's output with the urgent demand for granulocytes.
Under normal conditions, steady-state granulopoiesis maintains granulocyte levels to meet physiological needs. However, after a major insult, typically a bacterial infection, the hematopoietic program switches from steady-state to emergency granulopoiesis. This switch is mediated by a transition from C/EBPα to C/EBPβ, the primary transcriptional regulator of emergency granulopoiesis. C/EBPβ enhances the production of granulocytes by promoting the progression of the cell cycle of myeloid progenitors at an accelerated rate, thereby generating a sufficient amount of new granulocytes to combat the insult.
The transcription factor CCAAT/enhancer binding protein β (C/EBPβ) is a critical regulator in this context, significantly influencing granulocyte lineage commitment and proliferation, especially noted during candidemia-induced scenarios.
The genetic backdrop plays a crucial role in the dynamics of emergency granulopoiesis, as demonstrated by studies in TP53 haploinsufficient models, particularly in FANCC−/− mice, highlighting the intricate interplay between genetic predispositions and the granulopoietic response. Additionally, recent advances have highlighted the importance of both direct and indirect pathogen sensing mechanisms. HSPCs are equipped with pathogen recognition receptors (PRRs) like Toll-like receptors (TLRs), enabling them to initiate myeloid differentiation and proliferation upon detecting pathogen-associated molecular patterns (PAMPs).
Neutrophils, as primary effector cells of the innate immune defense, originate from HSCs through a series of differentiation stages. The emergency granulopoiesis significantly accelerates this differentiation process, ensuring a rapid replenishment of neutrophil populations in response to systemic inflammatory stimuli, thus maintaining immune homeostasis.
The clinical significance of understanding emergency granulopoiesis extends beyond basic science, influencing therapeutic strategies against infectious diseases and immune deficiencies. Balancing rapid neutrophil mobilization against the risk of immune dysregulation is critical, as imbalances can lead to severe conditions like acute respiratory distress syndrome and sepsis-induced organ dysfunctions.
References
Text submitted to CC-BY-SA license. Source: Granulopoiesis by Wikipedia (Historical)
Articles connexes
- Myeloblast
- Band cell
- Metamyelocyte
- Granulocyte
- Haematopoiesis
- Myelopoiesis
- Howell-Jolly body-like inclusions
- SFRS2
- Lactoferrin
- Cyclic neutropenia
- Inborn errors of carbohydrate metabolism
- Endothelial protein C receptor
- Lymphoid enhancer-binding factor 1
- Mitophagy
- Lymphocyte-variant hypereosinophilia
- DEFA1
- FANTOM
- Outline of immunology
- Ii antigen system
- Alpha defensin
Owlapps.net - since 2012 - Les chouettes applications du hibou